Product Overview
The STERRAD™ 100NX System with ALLClear™ Technology sterilizes medical devices by diffusing hydrogen peroxide vapor into the chamber and then electromagnetically exciting the hydrogen peroxide molecules into a low-temperature plasma state.
With over 22,000 STERRAD™ Systems installed globally, it is trusted by healthcare institutions around the world to terminally sterilize critical and semi-critical medical devices including various classes of flexible endoscopes.
ASP elevates the standard of care by bringing the first safe and effective hydrogen peroxide gas plasma sterilization method indicated to reprocess duodenoscopes to the US market.
Cycle Information
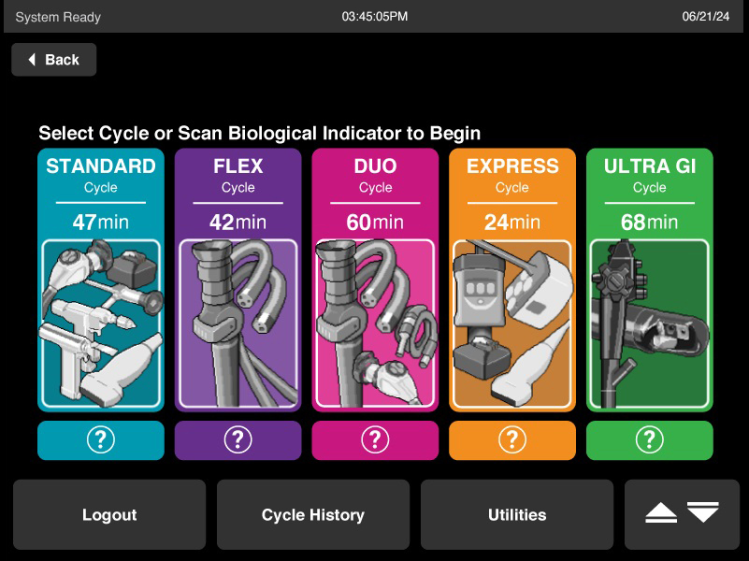
Includes up to five sterilization cycles – STANDARD, EXPRESS, FLEX, DUO, and ULTRA GI – to sterilize a variety of medical instruments, supported by endorsements from medical device manufacturers.
STANDARD Cycle: 47 Minutes*
- Single Channel Stainless Steel Lumens
- General Medical Instruments
Instruments include, But Are Not Limited To:
- Arthroscope And Laparoscopic Instrument Sets
- Eye Instruments
- Cystoscope Instruments
- Rigid And Semi-rigid Ureteroscopes
- Cameras And Light Cords
- Rechargeable Batteries
- Orthopedic Drills And Saws
- Ultrasound Probes/Transducers
*Cycle times are approximate. ALLClear™ Technology may increase processing time by approximately 5 minutes.
FLEX Cycle: 42 Minutes*
- Single Channel Flexible Endoscopes
- Flexible Endoscopes Without Lumens
Instruments include, But Are Not Limited To:
- Bronchoscopes
- Hysteroscopes
- Cystoscope
- Flexible Ureteroscopes
- Choledochoscopes
- Thoracoscopes
- Intubation Fiberscopes
*Cycle times are approximate. ALLClear™ Technology may increase processing time by approximately 5 minutes.
DUO Cycle: 60 Minutes*
- Single Channel Flexible Endoscopes
- Flexible Endoscopes Without Lumens
- Cameras
- Accessory Light Cords
Instruments include, But Are Not Limited To:
- Bronchoscopes
- Hysteroscopes
- Cystoscope
- Flexible Ureteroscopes
- Choledochoscopes
- Thoracoscopes
- Intubation Fiberscopes
- Light Cords
*Cycle times are approximate.
EXPRESS Cycle : 24 Minutes*
- General Medical Devices Requiring Surface
- Sterilization, Sterilization Of Mated Stainless Steel
- And Titanium Surfaces
Instruments include, But Are Not Limited To:
- da Vinci Endoscopes
- Rigid Or Semi-Rigid Endoscopes Without Lumens
- General Surgery Devices Without Lumens
- Rechargeable Batteries
- Eye Instruments Without Lumens
- Ultrasound Probes/Transducers
*Cycle times are approximate. ALLClear™ Technology may increase processing time by approximately 5 minutes.
ULTRA GI Cycle Processing: 68 Minutes*
ULTRA GI Cycle is designed for sterilization of the following:
- Hydrogen peroxide compatible flexible multi-channel duodenoscopes with no more than 4 channels, with lumen dimensions having an inside diameter (ID) of ≥1mm x ≤1500mm in length, or ≥2mm ID x ≤1630mm in length.
- One flexible duodenoscope per tray, and no more than 2 flexible duodenoscopes per cycle.
Note 1: The STERRAD 100NX Sterilizer ULTRA GI Cycle was validated using a load weight of 15.4 lbs (2 x 7.7 lbs), one endoscope per shelf.
Note 2: Only duodenoscopes that have been cleared as compatible with vaporized hydrogen peroxide are acceptable. Check STERRAD Sterilizer Cycle Selection table for ULTRA GI Cycle compatible duodenoscopes.
*Cycle times are approximate. Integrated ALLClear Technology, included in cycle time
STANDARD Cycle: 47 Minutes*
- Single Channel Stainless Steel Lumens
- General Medical Instruments
Instruments include, But Are Not Limited To:
- Arthroscope And Laparoscopic Instrument Sets
- Eye Instruments
- Cystoscope Instruments
- Rigid And Semi-rigid Ureteroscopes
- Cameras And Light Cords
- Rechargeable Batteries
- Orthopedic Drills And Saws
- Ultrasound Probes/Transducers
*Cycle times are approximate. ALLClear™ Technology may increase processing time by approximately 5 minutes.
FLEX Cycle: 42 Minutes*
- Single Channel Flexible Endoscopes
- Flexible Endoscopes Without Lumens
Instruments include, But Are Not Limited To:
- Bronchoscopes
- Hysteroscopes
- Cystoscope
- Flexible Ureteroscopes
- Choledochoscopes
- Thoracoscopes
- Intubation Fiberscopes
*Cycle times are approximate. ALLClear™ Technology may increase processing time by approximately 5 minutes.
DUO Cycle: 60 Minutes*
- Single Channel Flexible Endoscopes
- Flexible Endoscopes Without Lumens
- Cameras
- Accessory Light Cords
Instruments include, But Are Not Limited To:
- Bronchoscopes
- Hysteroscopes
- Cystoscope
- Flexible Ureteroscopes
- Choledochoscopes
- Thoracoscopes
- Intubation Fiberscopes
- Light Cords
*Cycle times are approximate.
EXPRESS Cycle : 24 Minutes*
- General Medical Devices Requiring Surface
- Sterilization, Sterilization Of Mated Stainless Steel
- And Titanium Surfaces
Instruments include, But Are Not Limited To:
- da Vinci Endoscopes
- Rigid Or Semi-Rigid Endoscopes Without Lumens
- General Surgery Devices Without Lumens
- Rechargeable Batteries
- Eye Instruments Without Lumens
- Ultrasound Probes/Transducers
*Cycle times are approximate. ALLClear™ Technology may increase processing time by approximately 5 minutes.
ULTRA GI Cycle Processing: 68 Minutes*
ULTRA GI Cycle is designed for sterilization of the following:
- Hydrogen peroxide compatible flexible multi-channel duodenoscopes with no more than 4 channels, with lumen dimensions having an inside diameter (ID) of ≥1mm x ≤1500mm in length, or ≥2mm ID x ≤1630mm in length.
- One flexible duodenoscope per tray, and no more than 2 flexible duodenoscopes per cycle.
Note 1: The STERRAD 100NX Sterilizer ULTRA GI Cycle was validated using a load weight of 15.4 lbs (2 x 7.7 lbs), one endoscope per shelf.
Note 2: Only duodenoscopes that have been cleared as compatible with vaporized hydrogen peroxide are acceptable. Check STERRAD Sterilizer Cycle Selection table for ULTRA GI Cycle compatible duodenoscopes.
*Cycle times are approximate. Integrated ALLClear Technology, included in cycle time

ASP innovates in partnership with device manufacturers to promote compliant reprocessing. ASP developed the ULTRA GI Cycle to sterilize duodenoscopes due to concerns and challenges with the current workflow and relatively high rates of contaminated endoscopes post-processing. ULTRA GI Cycle has integrated pre-load monitoring.
ASP is proud to bring this innovative solution to market with leading endoscope manufacturers to continue protecting patients during their most critical moments.
Future Focused System
Upgradeable technology that enhances system capability.
- An expandable, upgradeable sterilization system that enhances processing capability.
- Benefit from the latest technology while preserving the value of your capital investment.
- Maintain compliance as standards and regulatory guidance are updated.
- ULTRA GI Cycle will soon be available on all new STERRAD 100NX Sterilizers with ALLClear Technology and as an upgrade to
your existing system.
Trusted Sterility
Sterility assurance from a leader in low-temperature sterilization.
- Pioneers in vaporized hydrogen peroxide and hydrogen peroxide gas plasma sterilization technology for over 25 years.
- Over 15 million cycles successfully completed worldwide each year, impacting millions of patients annually.
- Over 20,000 medical device manufacturer endorsements.
- The ALLClear™ Technology within the system increases sterilization productivity by conditioning the load, checking for moisture,
and running system diagnostics prior to sterilization cycles to maximize successful cycle runs.
Preserve & Improve Margin of Safety
Innovation focused on patient protection.
- Terminal sterilization offers an improved margin of safety versus other reprocessing modalities, such as high-level disinfection and liquid chemical sterilization.
- ULTRA GI Cycle offers this improved margin of safety for hydrogen peroxide compatible duodenoscopes.
Designed to minimize healthcare worker chemical exposure.
- Touch-free cassette disposal promotes your team’s safety.1
- The Hydrogen peroxide gas plasma sterilization technology helps enhance the safety of patients and sterile processing staff. Gas plasma is documented to actively break down hydrogen peroxide, reducing emissions exposure for sterile processing staff.2
Reduced Cost
Delivers ongoing economic value.
- Proven to preserve instrument integrity, reducing the frequency of costly repairs and replacements compared to steam sterilizers.3-4
- Consume approximately 70% less energy per year than steam sterilizers.5
- Use of STERRAD™ Systems instead of steam sterilizers can save an estimated 180,000 liters of water per year.5
Specs
1-Door system
Dimensions:
30.5 in (77.5 cm) W x 70.9 in (180.0 cm) H x 41.5 in. (105.4 cm) D
Weight:
842 lbs (382 kg)
2-Door system
Dimensions:
30.5 in (77.5 cm) W x 70.9 in (180.0 cm) H x 43.1 in (109.5 cm) D
Weight System:
900 lbs (408 kg)
Resources
User's Guide
The ASP IFU and User Guide library allows users to search by language, country and product to locate the appropriate instructions for use of ASP Products. Product literature and availability varies by country. Check out this helpful reference site to locate the STERRAD™ 100NX System with ALLClear™ Technology instructions for use.
View and download User's Guide
Device Validations and Compatibility
The STERRAD™ Sterility Guide (SSG) is an easy to use, first of its kind online tool designed to provide STERRAD™ System customers with an up-to-date list of devices that fall within STERRAD™ Systems claims for sterility.

ASP is pleased to offer medical equipment financing options through our business relationships with leading financial institutions.
Equipment financing options include*:
PAYMENT TERMS TO FIT WITHIN YOUR BUDGET • RENT-TO-OWN • DEFERRED PAYMENTS
Equipment financing options include*:
- PAYMENT TERMS TO FIT WITHIN YOUR BUDGET
- RENT-TO-OWN
- DEFERRED PAYMENTS
*Financing options are subject to change and may vary based on numerous factors. The third-party trademarks used herein are the properties of their respective owners. ASP is not affiliated with the third-party lender or any of its subsidiaries or its affiliates. Any transaction is between the customer and lender. ASP is only acting as a referral source.
References
- As a precaution, when handling any part of the system or load items that have been exposed to hydrogen peroxide, please wear the appropriate PPE (chemical-resistant latex, PVC/vinyl or nitrile gloves). Refer to the glove manufacturer's instructions for use for more information.
- Comparison Study of Environmental Hydrogen Peroxide Levels of STERRAD® Systems and STERIS® V-Pro® Low Temperature Sterilizers Reveal Striking Differences. The research was designed and executed by Actionable Research, an independent third-party research firm in conjunction with ChemDAQ® Inc., a manufacturer of environmental. Safety monitoring systems. The research sponsor was Advanced Sterilization Products. All data were collected by the ChemDAQ® staff.
- Schäfer, B. Decreased number of repairs of rigid scopes as a result of low-temperature sterilization with H2O2 gas plasma. International Journal of Sterile Supply.2009;17(3):194-196.
- Skogås, JG. Effects of sterilisation methods on rigid endoscopes.Trondheim, Norway: Medical Technology Department, Trondheim University Hospital;1999
- Comparing the Environmental Impacts of Sterrad Sterilization with Competing Approaches Irvine, CA: Advanced Sterilization Products; 2016. Research Sponsored by ASP.