Product Overview
Elevate the Standard of Care
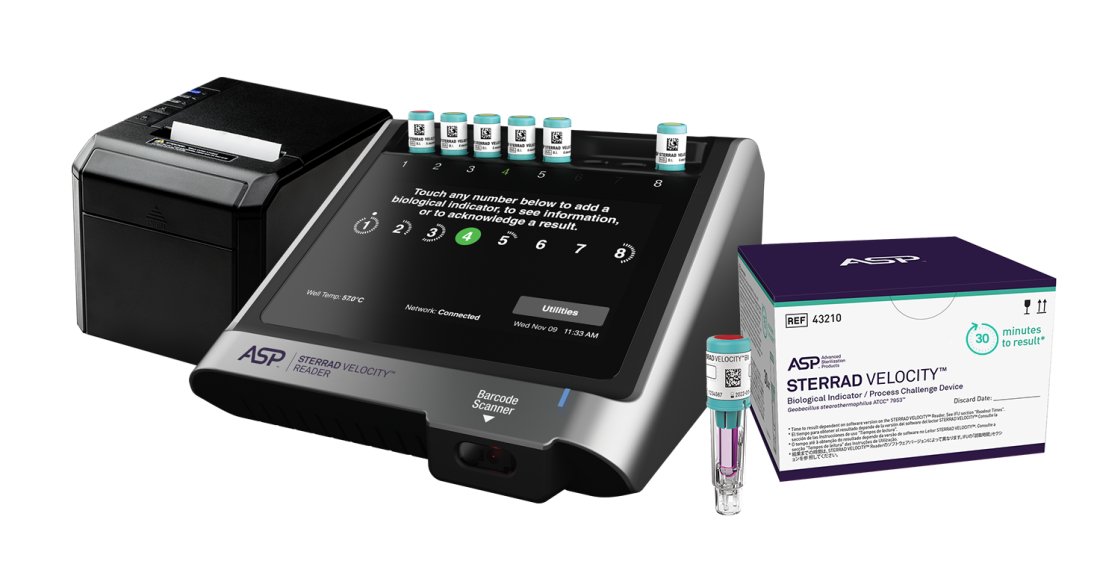
Be confident in the sterility assurance of your instruments before they are used on patients. The confidence that only comes from meeting the elevated standard of AAMI guidelines (ST58:2013/(R)2018)2, together with STERRAD™ System process monitoring accuracy.
- STERRAD VELOCITY™ BI/PCD provides the protection of the rapid read Process Challenge Device (PCD) for STERRAD™ Systems that meets the elevated standard of AAMI recommended guidelines (ST58 9.5.4.3)
- The self-contained Process Challenge Device (PCD) for STERRAD™ Systems that provides sterility assurance through a resistance greater than or equal to the most challenging hospital-defined load (ST58 9.5.4.1)
- The all-in-one Process Challenge Device designed by ASP specifically for all STERRAD™ System Cycles to minimize false positives
Software
IFU
The ASP IFU and User Guide library allows users to search by language, country and product to locate the appropriate instructions for use of ASP Products. Product literature and availability varies by country. Check out this helpful reference site to locate the STERRAD VELOCITY™ Biological Indicator / Process Challenge Device instructions for use.
View and download instructions for use (IFU)
References
- 15 or 30 minutes to result dependent on the software version on the STERRAD VELOCITY™ Reader. 15 minutes to result for SW version 1139260417 or greater; 30 minutes to result for SW version 1139260317 or below.
- ANSI/AAMI ST58 2013/(R)2018. Chemical Sterilization And High-Level Disinfection in Health Care Facilities (ST58).