Overview
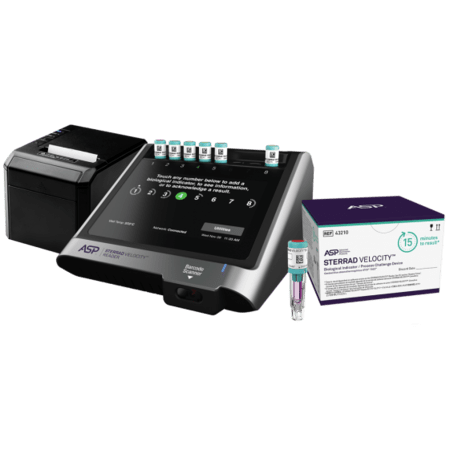
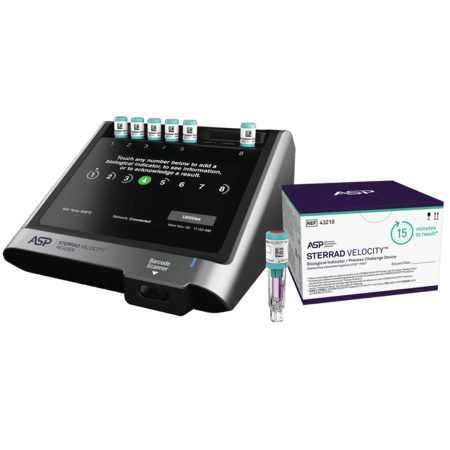
The STERRAD VELOCITY™ System comprises of:
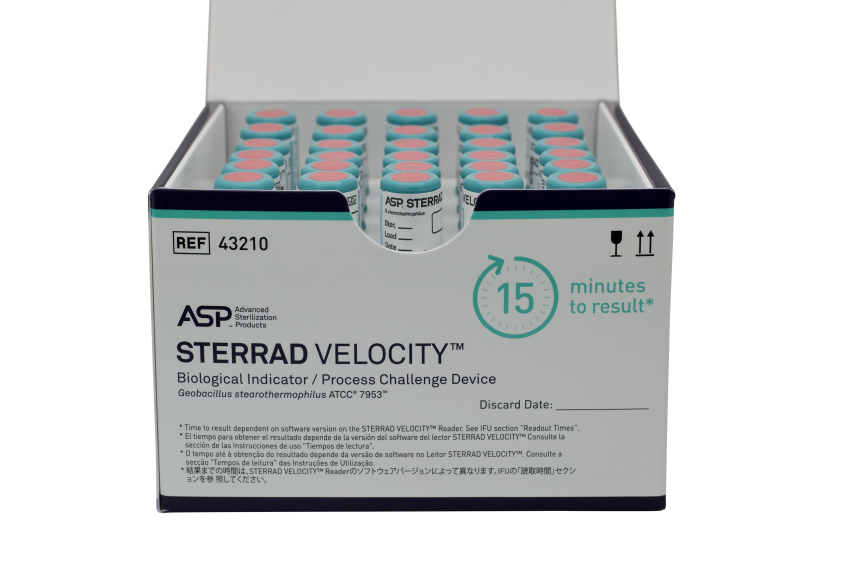
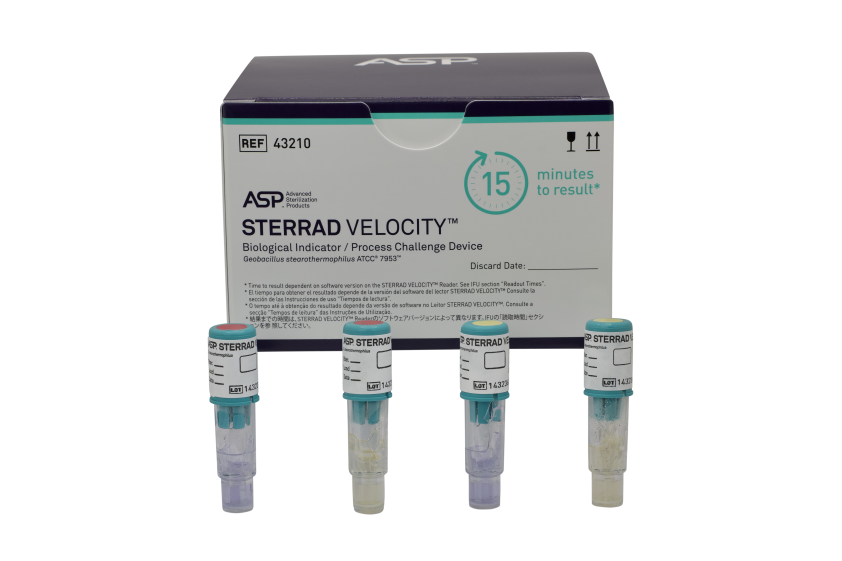
- the STERRAD VELOCITY™ Biological Indicator/Process Challenge Device (BI/PCD)
- the STERRAD VELOCITY™ Reader
It is manufactured specifically for use in STERRAD™ Systems and is the only BI/PCD validated by ASP.
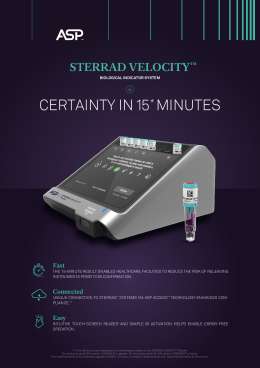
Our BI/PCD is an easy-to-use, self-contained that reads with certainty, providing fast and efficient sterility assurance to help improve instrument turnaround time.1, 2
It is the only BI/PCD system that communicates with STERRAD™ sterilizers, thereby enabling the automatic documentation of each cycle and allowing users to quickly and compliantly carry out BI processing, to produce audit-ready records.2
Both STERRAD VELOCITY™ BI/PCD and Reader are intended to be used for:
- Frequent monitoring and periodic testing of STERRAD™ Sterilization Systems and their respective cycles (at each cycle or at defined periodicity according to applicable recommendations and standard operating procedures for hospitals)1
Qualifications of STERRAD™ Systems after installation, sterilizer relocation, major repairs or for periodic requalification according to ISO 149371
For qualifications, STERRAD VELOCITY™ BI/PCD is used in conjunction with reference loads from the STERRAD VELOCITY™ System Validation Kit, which are defined for each STERRAD™ System and cycle1
STERRAD VELOCITY™ Biological Indicator is the suggested product replacement for CYCLESURE™ 24 Biological Indicator (BI).
Features
Rapid BI readout
- Read time of 15-minutes3* – allows users to know the result before they release the load
- Reduces instrument turnaround time, helping users to meet the demands of OR schedules2
- Built-in chemical indicator allows for quick identification of processed versus unprocessed BIs2
- Multiple wells in the reader3 facilitate its use in larger hospitals which use multiple STERRAD™ Systems
All-in-one BI and PCD combined device
- STERRAD VELOCITY™ is both a Biological Indicator as well as a Process Challenge Device, elevating the sterilization process monitoring standard.
- Biological Indicators and Process Challenge Devices represent different levels of challenge in the sterilization process. They are intended to demonstrate whether the conditions were adequate to achieve sterilization and, in general, do not have the requirement to represent worst-case devices per ISO 11138 and FDA guidance.
- Process Challenge Devices provide a challenge to the sterilization process that is equal to or greater than the worst-case medical device loads routinely processed.
- STERRAD VELOCITY™ is the only all-in-one BI/PCD that meets AAMI guidelines and other global standards and provides sterility assurance by providing a resistance greater than the worst-case hospital loads.
- The STERRAD VELOCITY™ BI / PCD System is fully validated with STERRAD™ Systems and ensures that only medical devices with assured sterility reach the patient, helping to minimise the risk of Hospital Acquired Infections, HAI.
Reads with Certainty
- Uses advanced optical measurement technology to detect biological activity2
- Eliminates subjectivity seen with visual interpretation of results2
Streamlines BI Processing and Record-Keeping
- Only releases instruments when they are ready, reducing the risk of releasing instruments prior to BI confirmation2, 3
- Automatic record keeping provides consistent and complete BI records, aiding compliance2
- Compatible with ASP ACCESS™ Technology, allowing automatic correlation of STERRAD™ System cycle records and BI results2
- On-board barcode scanner eliminates the need for manual data entry, minimising the risk of error2
User-friendly
- Intuitive touch-screen interface provides step-by-step instructions, reducing user errors2
- BI results are displayed clearly and easy-to-read countdown timers are shown for each well2
- On-screen, audible alerts update staff with critical information2
BI Specifications
The STERRAD VELOCITY™ BI/Process Challenge Device contains two important components required for fluorescence readout:
- α-MUG: a fluorogenic dye activated upon enzymatic reaction with α-glucosidase (AG)
- α-glucosidase (AG): a natural enzyme, produced by spores
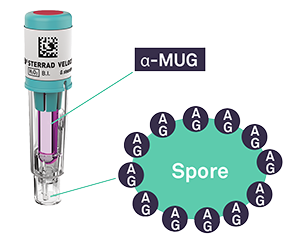
When the ampule is crushed, the surviving microorganisms, containing AG, are exposed to a media containing α-MUG.
The resulting enzymatic reaction splits α-MUG into 4-MU, a fluorescent compound, and glucose.
If microorganisms survive post-sterilization, the media will fluoresce, indicating a positive BI. Alternatively, in the case that no microorganisms survive after the sterilization cycle, the media will not fluoresce and the BI can be considered negative.
This advancement in readout technology enables sterile processing leaders to establish a greater balance between scaling to clinical demand and increasing the confidence in BI/PCD results prior to releasing instruments.
Software
ASP ACCESS™ Technology is a smart information-sharing technology that seamlessly connects, correlates, and communicates vital reprocessing information across ASP devices and hospital network systems.4 It works to enhance compliance through reducing the risk of human error, and optimise reprocessing efficiency through automation of record-keeping and the provision of performance data.4
- Enables seamless communication between STERRAD™ Systems, STERRAD VELOCITY™ , instrument tracking systems (ITS) and hospital networks5
- Automatically correlates sterilization cycles and BI results to produce audit-ready data4
- Cloud technology enables staff to access reprocessing records from any location and receive alerts via email/text4
Product Name | Product Number | Packaging |
|---|---|---|
STERRAD VELOCITY™ Reader | 43220 | 1 piece |
STERRAD VELOCITY™ Biological Indicator/Process Challenge Device (BI/PCD) | 43210 | 2 pouches, 30 BIs each |
STERRAD VELOCITY™ Biological Indicator/Process Challenge Device (BI/PCD) - Outside US only | 43210-30 | 1 pouch, 30 BIs |
Printer | 43221 | 1 piece |
Hand-held scanner | 10308 | 1 piece |
IFU
The ASP IFU and User Guide library can be used to search for the appropriate IFU, User Guides and Data Sheets for ASP products. Product literature and availability varies by country; users can ensure they locate the correct literature for their setting by searching by product and country. Use the link below to locate the STERRAD VELOCITY™ IFU.
References
- Advanced Sterilization Products. STERRAD VELOCITY™ BI/ PCD Technical Dossier.
- Advanced Sterilization Products. STERRAD VELOCITY™ Biological Indicator System Product Brochure. AD-170021-01-CT_C-MDR.
- Advanced Sterilization Products. STERRAD VELOCITY™ Biological Indicator System Data Sheet. AD-170020-01-CT_C-MDR.
- Advanced Sterilization Products. ASP ACCESS™ Technology Product Brochure. AD-160030-01-CT_C-MDR.
- Advanced Sterilization Products. ASP Ecosystem Brochure - Innovation to Advance the Quality of Patient Care. AD-170035-01-CT_D-MDR.