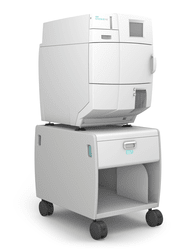
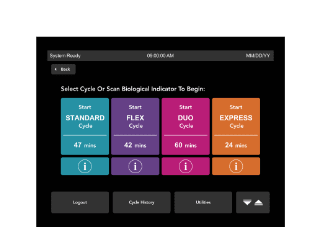
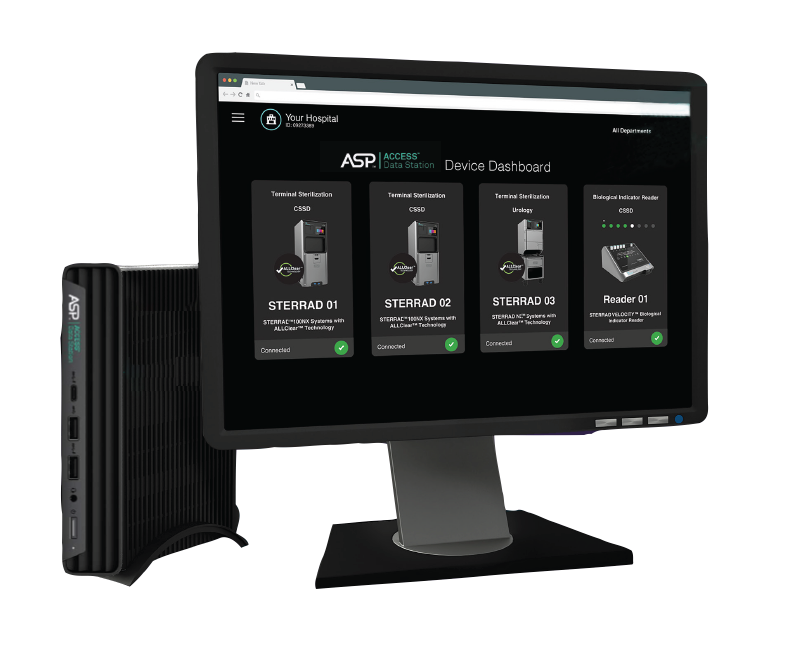
Future-proof your Sterile Processing Department
An increasing number of healthcare procedures are occurring in outpatient, ambulatory care.1 Processing surgical instruments is at the heart of every surgery center. The Sterile Processing Technicians are at the helm of driving patient care during surgery by being responsible for cleaning, decontaminating, processing, sterilizing, and managing the necessary instruments and equipment. Their dedication and diligence can often be challenged when facilities aren’t efficiently equipped with the appropriate hardware and tools that allow for a scalable workload.
Developing methods to minimize shocks and stresses of foreseeable future events is important.
Is your business or team growing, adding procedures, driving efficiencies or investing in robotics? Get a consultation today from an ASP expert on how ASP technology can help future-proof your SPD.