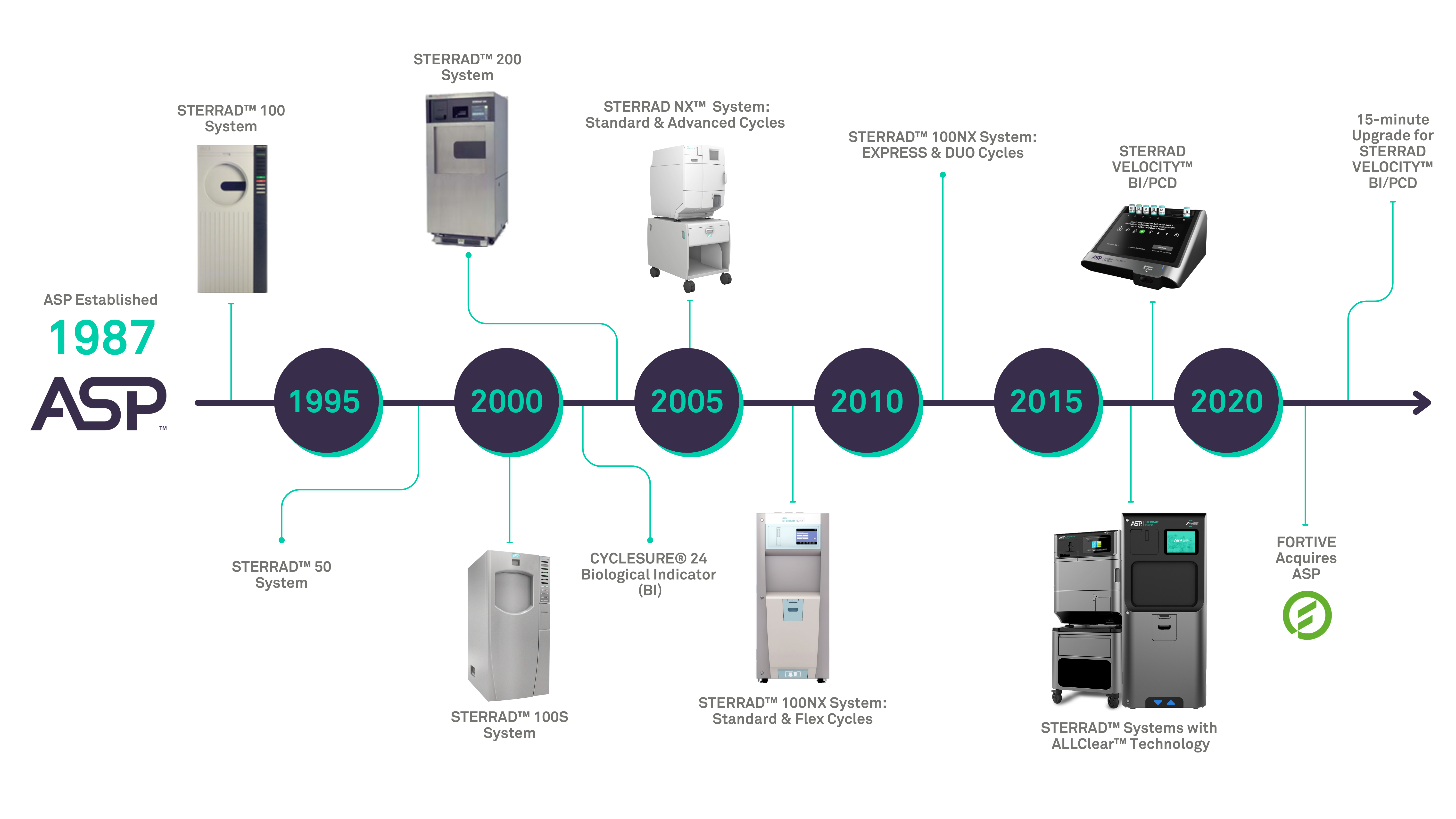
OUR MISSION
Protecting patients during their most critical moments.™
Every day, millions of people around the world visit a healthcare facility. The care and handling of instruments used to treat patients is vital to their health outcome, and there is no room for error.
Advanced Sterilization Products is a leader in infection prevention, dedicated to creating the products, solutions, and processes needed by practitioners to protect patients during their most critical moments. Our ultimate responsibility is to the patient—it is our job to elevate the standard of care for patients everywhere, and we are committed to continuously advancing infection prevention technologies that healthcare depends upon.