Understanding the Difference¹
Disinfection
Sterilization
Reprocessing Modality
| Organism | Sterilization |
|---|---|
|
Most Difficult to Kill |
|
| Bacterial Spores | |
| Mycobacteria | |
| Fungi | |
| Vegetative Bacteria | |
| Enveloped Viruses |
Less Difficult to Kill
| Organism | High-Level Disinfection (HLD) |
|---|---|
|
Most Difficult to Kill |
|
| Bacterial Spores | X |
| Mycobacteria | |
| Fungi | |
| Vegetative Bacteria | |
| Enveloped Viruses |
Less Difficult to Kill
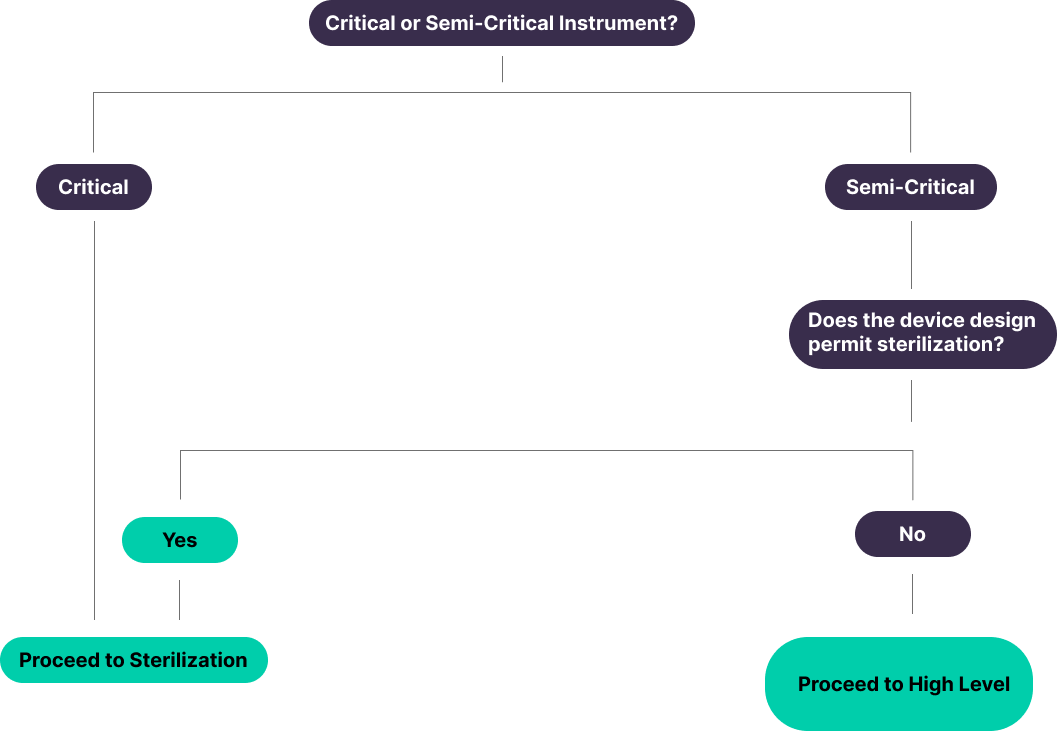
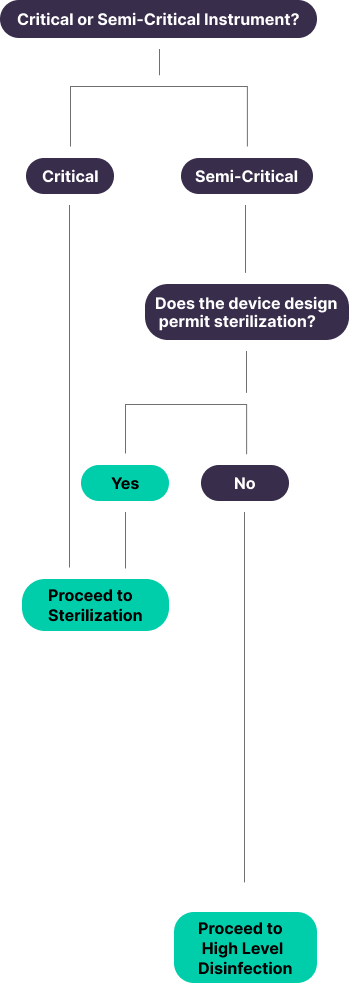
Classification and Examples of Medical Devices and/or Equipment²
Critical Items
Semi-Critical
Shift to Sterilization

AAMI TIR68: 2018 (R2022)3
“Semi-critical devices are devices that contact intact mucous membranes or non-intact skin. Users should be instructed to thoroughly clean these devices and then reprocess them by sterilization. If the device design does not permit sterilization (e.g., device materials cannot withstand sterilization), then high-level disinfection should be used.”
STERRAD™ Systems can sterilize a wide-range of instruments
For a list of model numbers that can be sterilized in STERRAD™ Systems, please visit the STERRAD™ Sterility Guide at www.sterradsterilityguide.com.
Request a consultation today with one of our sterilization experts.
References
Centers for Disease Control and Prevention (CDC). Introduction, Methods, Definition of Terms -Guideline for Disinfection and Sterilization in Healthcare Facilities (2008) https://www.cdc.gov/infectioncontrol/guidelines/disinfection/introduction.html.
Centers for Disease Control and Prevention (CDC). A Rational Approach to Disinfection and Sterilization - Guideline for Disinfection and Sterilization in Healthcare Facilities (2008). https://www.cdc.gov/infectioncontrol/guidelines/disinfection/rational-approach.html.
American National Standard/Association for the Advancement of Medical Instrumentation. ANSI/AAMI TIR68:2018 (R2022) Low and intermediate-level disinfection in healthcare settings for medical devices and patient care equipment and sterile processing environmental surfaces.
Association of periOperative Registered Nurses (AORN). 6 Dos and Don’ts for Sterile Processing in ASCs. September 25, 2019. https://www.aorn.org/article/2019-10-22-Sterile-Processing-in-ASCs
*ASP AEROFLEX™ AER and EVOTECH® ECR have been cleared by United States Food and Drug Administration (FDA) to high-level disinfect flexible, semi-critical endoscopes.
**Does not eliminate bedside cleaning and may not eliminate manual cleaning; Health Care facilities should follow their own policies and procedures related to the reprocessing of endoscopes to ensure they are complying with all steps recommended by the device manufacturers and are consistent with current standards and guidelines. Not all endoscopes can be automatically cleaned but may be high-level disinfected. It is recommended that endoscopes with open/closed elevator wire channels be manually cleaned as per manufacturer's instructions in addition to using the cleaning cycle of the EVOTECH® System. Please refer to the EVOTECH® ECR User Guide and specific connection diagrams for more detailed information regarding cycle capabilities.
The third-party trademarks used herein are the properties of their respective owners.