H2O2
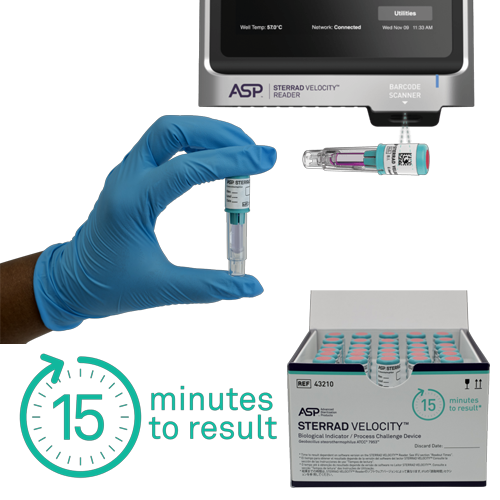
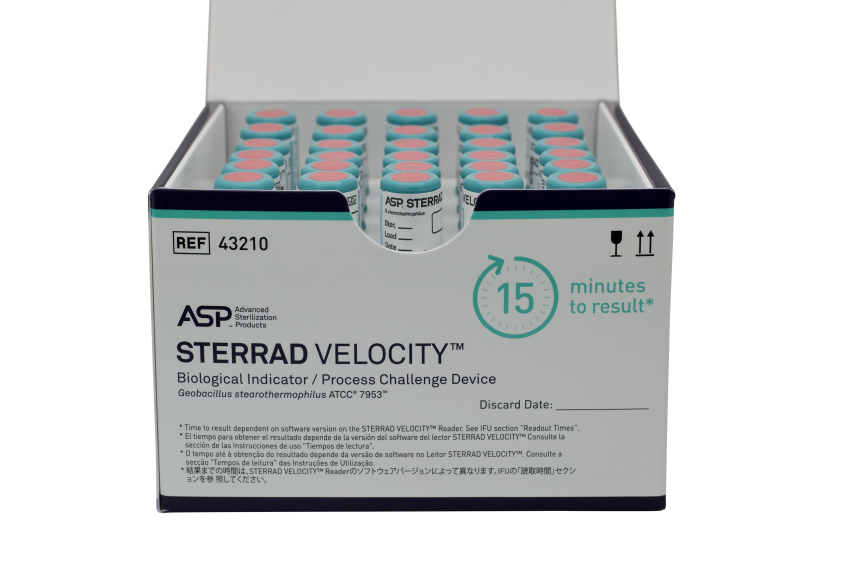
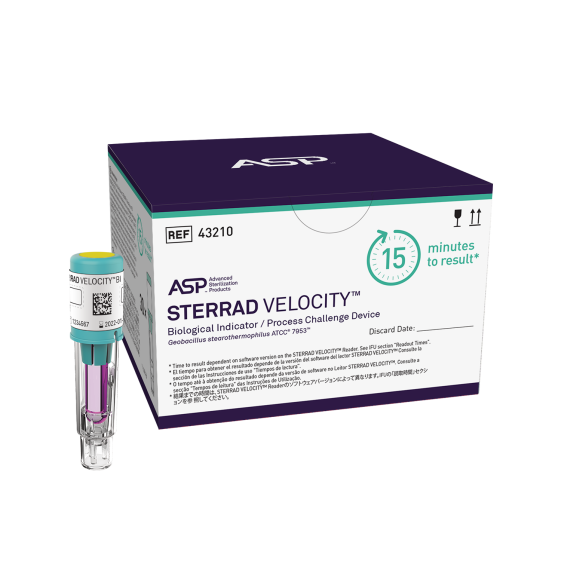
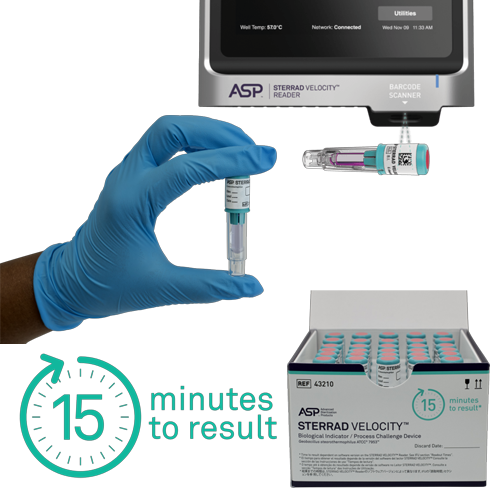
STERRAD VELOCITY™ is both a Biological Indicator as well as a Process Challenge Device, elevating the sterilization process monitoring standard.
Biological Indicators and Process Challenge Devices represent different levels of challenge in the sterilization process.
They are intended to demonstrate whether the conditions were adequate to achieve sterilization and, in general, do not have the requirement to represent worst-case devices per ISO 11138 and FDA guidance.
STERRAD VELOCITY™ is the only all-in-one BI/PCD that meets AAMI guidelines and other global standards and provides sterility assurance by providing a resistance greater than the worst-case hospital loads.
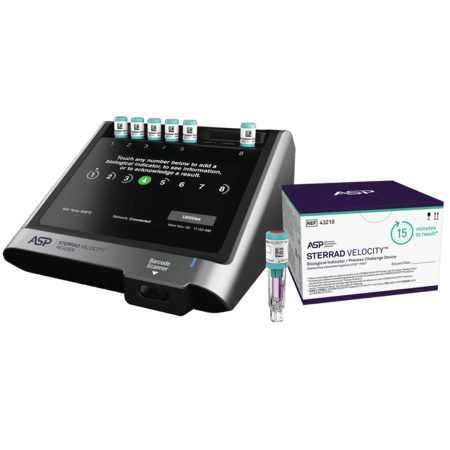
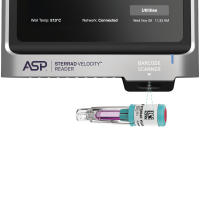
The STERRAD VELOCITY™ BI/PCD System is used together with STERRAD™ Velocity Reader is fully validated with STERRAD™ Systems and ensures that only medical devices with assured sterility reach the patient, helping to minimise the risk of Hospital Acquired Infections, HAI.
Product Name | Product Number | Packaging |
|---|---|---|
STERRAD VELOCITY™ BI/PCD | 43210 | 2 pouches, 30 BIs each |
STERRAD VELOCITY™ BI/PCD - Outside US only | 43210-30 | 1 pouch, 30 BIs |
STEAM
| Product Name | Product Number | Packaging |
|---|---|---|
| BIOTRACE™ Auto Read Pro Reader | 73400 | 1 each |
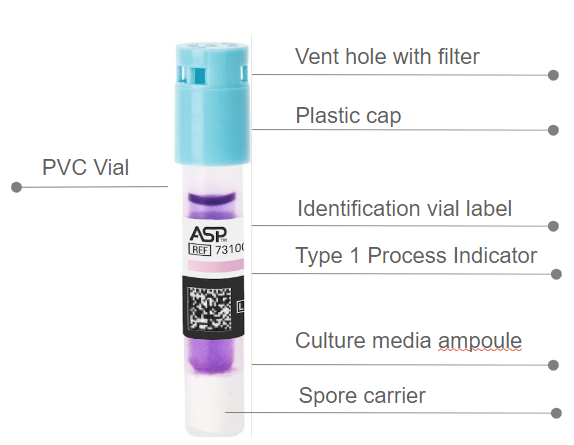
| BIOTRACE™ Auto Read 20 Steam BI | 73100 | 50 BIs per box / 2 boxes per case |
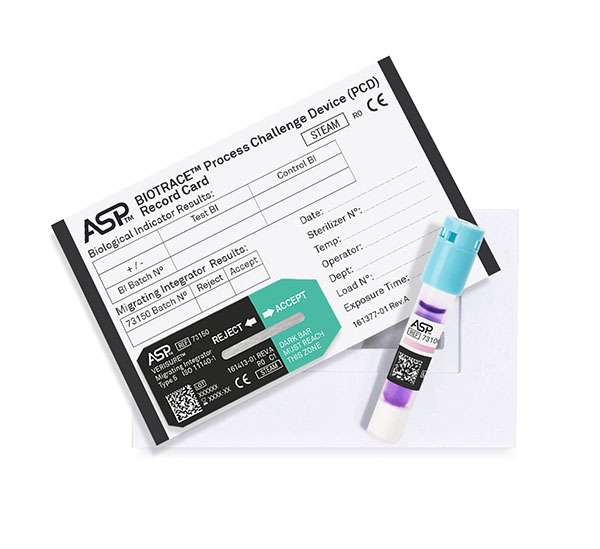
| BIOTRACE™ Auto Read 20 Steam BI Process Challenge Device (PCD) Kit | 73130 | 25 kits per case |
| Thermal Printer Paper (Pro Reader, 50 mm diameter) | 73451 | 6 rolls per case |
Product Name | Product Number | Packaging |
|---|---|---|
BIOTRACE™ Auto Read 60 Steam BI | 73105 | 50 BIs per box / 2 boxes per case |
BIOTRACE™ Auto Read 60 Steam BI/PCD | 73135 | 25 PCDs + 25 Controls per case |
Product Name | Product Number | Packaging |
|---|---|---|
BIOTRACE™ Instant Read Steam Biological Indicator | 73110 | 50 BIs per box / 2 boxes per case |
BIOTRACE™ Instant Read Steam BI Process Challenge Device (PCD) | 73140 | 25 PCDs + 25 Controls per case |
EtO
Product Name | Product Number | Packaging |
|---|---|---|
BIOTRACE™ Auto Read 4H EO Biological Indicator | 73300 | 50 BIs per box / 2 boxes per case |