In the modern healthcare environment, the importance of preventing outbreaks and ensuring the safe reprocessing of medical instruments is paramount. Healthcare-associated infections (HAIs o HCAIs) continue to pose significant risks, making the implementation of effective prevention and reprocessing methods crucial for patient and staff safety and healthcare efficiency.
The CSSD is constantly under pressure to enhance turnaround times, reduce costs, and optimize workflow efficiency to maximize device availability for healthcare professionals and patients. The reprocessing of medical devices has significant cost and resource implications within the CSSD, ultimately affecting the efficiency of patient care within the hospital.
Our ebook “Efficiency in the CSSD” goes through the best practices for preventing outbreaks of hospital acquired infections and ensuring the thorough reprocessing of medical devices. It provides a detailed overview of the latest standards, methods, and technologies that are transforming how healthcare facilities approach infection control.
By focusing on both prevention and the sterilization of instruments, I can help healthcare professionals reduce the incidence of HAIs, safeguard patient health, and comply with stringent regulatory requirements.
What’s in it for you?
- Enhanced Patient Safety: by implementing the strategies and techniques discussed, you can ensure that all medical instruments meet the highest standards of cleanliness and safety, significantly reducing the risk of healthcare-associated infections (HAIs) and safeguarding patient health.
Cost Savings: addressing inefficiencies in medical device reprocessing can lead to substantial cost savings. By optimizing workflow efficiency and reducing resource use, the healthcare facility can lower operational costs while maintaining high standards of care.
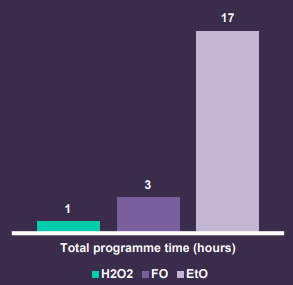
- Improved Turnaround Times: insights into advanced reprocessing techniques and technologies that can help you improve the turnaround time of medical devices (comparing EtO, FO and H2O2). This means quicker availability of sterile devices for surgical procedures, enhancing overall patient care.
- Regulatory Compliance: Stay ahead of stringent regulatory requirements by adopting the latest standards, methods, and technologies in infection control.

